BIGCS Data Access Policy
1. The scope of data sharing
The data in the Born in Guangzhou Cohort Study (BIGCS) include shareable and unshared data based on the relevant laws and regulations of the People's Republic of China. This file is applicable to shareable data.
2. The qualification for applicants:
(1) The applicants must be affiliated with a research institution that has a sound data security guarantee and usage regulation.
(2) The applicants must have a medical education background and must have a doctoral degree (Ph.D.) or equivalent research competence (eg. associated professor or above).
(3) As a first author or corresponding author, the applicants have published 2 or more papers in the journals with impact factors >10.
(4) As a principal investigator (PI), the applicants must have led and completed at least one national or higher-level scientific research project and obtained research funding exceeding 1 million yuan (RMB).
3. Principles of data sharing
On the premise of meeting the qualification requirements for applicants, the data resources of this research project are shared based on the following principles:
(1) Data sharing, usage, application of related scientific research achievements, and publication of scientific research papers must be subjected to relevant laws, regulations, and administrative rules of the People's Republic of China.
(2) The data sharing must be based on a project collaboration between the applicants and Guangzhou Women and Children's Medical Center, Guangzhou Medical University (GWCMC). The collaboration project needs to obtain approval from the Ethics Committee of GWCMC. International collaboration projects should be registered in the international collaboration registration system of the Ministry of Science and Technology of China and be reviewed by the Ministry of Science and Technology of China.
(3) The data is for medical research purposes only, and the research content must be related to disease or health and cannot be used for commercial purposes.
(4) The data sharing encourages high-quality research collaboration and prioritizes the research conducted by Chinese scholars.
(5) No conflicts with ongoing or planned projects of GWCMC.
(6) Data can only be obtained by applying for and signing the Collaboration Agreement, Confidentiality Agreement, and Data Resource Use Agreement. Data users must have the ability to process and analyze the data they are applying for and strictly abide by the scope, research duration, and personnel specified in the agreement.
Unauthorized use of project data for any other project or purpose is prohibited. The researchers are not entitled to republish or otherwise make available any original data of BIGCS including sequencing data, analytical data, or derived variable data at the individual participant level in any way.
(7) The applicants should adhere to the ethical principles and protect research subjects under the principles of informed consent and privacy protection. The shared data should not contain the personal identity information of the research subjects. All data will be shared anonymously, and researchers are not allowed to identify and investigate research subjects through shared data.
(8) Data applicants, users, and other relevant personnel shall assume the responsibility for ensuring data confidentiality and security. They must adhere to the Regulations of the People's Republic of China on the Management of Human Genetic Resources, Biosecurity Law of the People's Republic of China, Cybersecurity Law of the People's Republic of China, Data Security Law of the People's Republic of China, Personal Information Protection Law of the People's Republic of China, and other applicable laws and regulations. The acquired data shall not be utilized for activities that jeopardize China's national security or social interests or infringe upon others' legitimate rights and interests. Failure to comply may result in accountability if serious consequences arise.
(9) The data users must adhere to the guidelines of scientific ethics, comply with international and national norms for conducting scientific research activities, and meet the requirements of research integrity. They are prohibited from engaging in plagiarism or misappropriation of others' research findings, as well as falsification or manipulation of research data and conclusions. Additionally, they are not allowed to engage in any other behaviors that violate the principles of research integrity.
(10) As the collector and manager of data, GWCMC has the ownership of the sharing data and is responsible for maintaining and preserving data and has the authority to control and manage the use of shared data.
(11) Publications and dissertations published using shared data should clearly state that the data are from BIGCS at GWCMC.
(12) Before the conclusion of the research project, the researcher must provide the database generated based on shared data (including derived variables) and variable descriptions back to GWCMC may in turn make available to other (approved) researchers.
4. Data sharing management department
The Ethics Committee, Academic Committee, and Data Management Committee of GWCMC are jointly responsible for data applications and management . The Ethics Committee and academic committee are responsible for approving data applications. The Data Management Committee is responsible for initial review, agreement signing, data extraction and transfer, and project progress tracking.
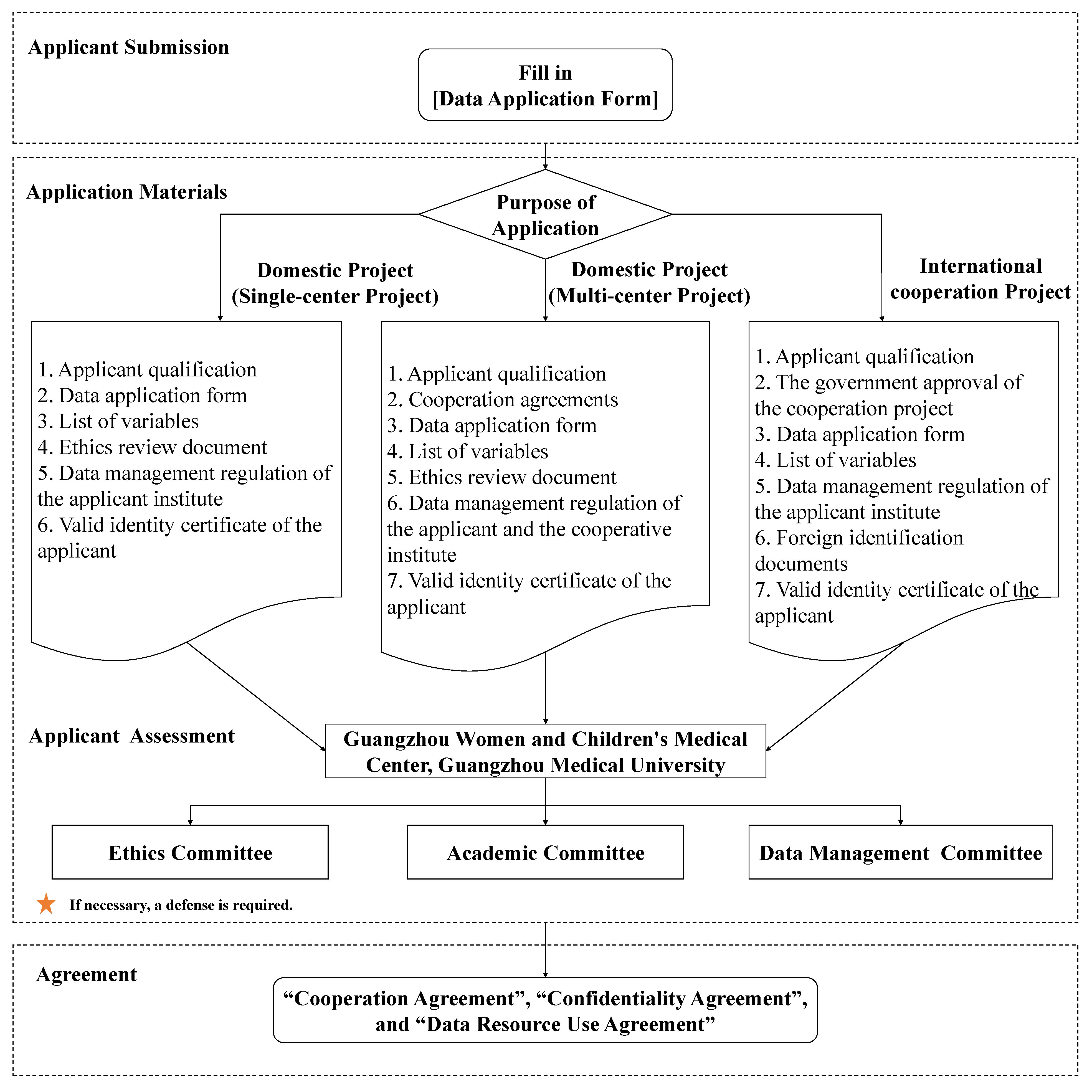
5. Data application process
The Ethics Committee, Academic Committee, and Data Management Committee are in charge of the assessment of the data application.
(1) Applicant submission
Submit the complete materials for application to the Data Management Committee via email, including the proof of the applicant's qualification, data application form, variable list, ethics review document, data management regulation in the research institution of the applicants affiliated with, valid identity certificate, and photocopy of the applicants. If it is an international collaborated project with the BIGCS, government approval for the collaboration shall be added. The Data Application Form and Variable List templates will be sent via Email. The variables should not be applied beyond the research purpose. A new application needs to be submitted if need to apply additional variables in the same project.
(2) Applicant assessment
The Data Management Committee conducts a preliminary review of the application materials. Complete application materials shall be submitted to the Ethics Committee and Academic Committee after being reviewed and approved by the Data Management Committee. Secondary approval shall be made by external experts. The external experts will review the scientific and feasibility of the research project, the applicant's research ability and funding situation, ethics, data confidentiality, and security. Applicants are required to conduct project defense when necessary.
(3) Sign up an agreement
After the application is approved, the applicant must sign a "Collabroation Agreement", "Confidentiality Agreement", and "Data Resource Use Agreement" with GWCMC, detailing the jointly agreed collabroation terms and data use terms, and defining the rights and obligations of both parties to the agreement. Applicants should fulfill their obligations to clean up data, save data, prevent data leakage, do not change data, and exchange experimental and data analysis results.
(4) Data extraction and transfer
After signing the aforementioned Agreements, the Data Management Committee will initiate an internal data outbound review process, and extract and generate anonymous datasets and related explanatory documents according to the variable list. A authorized account will be provided for downloading the data within a limited period. If the data already exists on the national data-sharing platform, it will be released through the platform. After receiving the data, the applicant needs to provide a signed data received form or feedback data issues via email within 15 days. Failure to provide feedback on data issues within the specified time limit requires resubmission of the application.
6. Process after data acquisition
After successfully acquireing the sharig data , the applicant must regularly report the progress of the project. The management committee requires applicants to submit interim reports according to project requirements every six months after the start of the project and submit summary reports, final databases (including Derived Data Variables), and variable descriptions two weeks before the end of the project.
7.Termination of data sharing
Upon approval of data sharing, the management committee reserves the right to unilaterally terminate such sharing if the applicant is found to be
(1) in violation of any signed agreements,
(2) engaged in academic misconduct or falsification,
(3) demonstrates evident analytical errors in experimental results and data analysis results while refusing to make necessary modifications upon notification,
(4) fails to submit interim reports on two consecutive occasions after obtaining the data,
(5) or other circumstances deemed necessary by the management committee.
Contact us
Email: data.bigcs@bigcs.org
Contact address: No. 9 Jinsui Road, The Born in Guangzhou Cohort Study Department, Guangzhou Women and Children's Medical Center, Guangzhou Medical University, Zhujiang Newtown, Guangzhou


